Introduction
As we emerge from the COVID-19 pandemic and prepare to return to offices, restaurants, retail shopping and all other places associated with “normal” life, it will be important to assure
employees and customers that they are not only welcome back, but that it is also safe for them to return. People will have concerns and proving that your cleaning and sanitation is effective and is providing them with an environment that is clean and safe will be an important step in welcoming them back. Evaluation of your cleaning methods in a way that provides proof will be a necessary first step.
So, how can that be done? We can learn from the cleaning practices in environments where bacteria, viruses and other contamination and infectious agents are a crucial concern and that have always been conducted using highly specific processes verified by analytical testing. Cleaning in hospitals for the prevention of healthcare-associated infections, for example, has typically employed ATP hygiene monitoring tests to assess the effectiveness of the cleaning procedures used. Cleaning in commercial and retail settings has traditionally been far less specific and precise, but this may no longer be an option for responsible site managers.
So what is an ATP Test?
ATP tests are easy-to-use, rapid tests that are used to assess the effectiveness of cleaning in a wide variety of industries and facilities, including food processing plants, hospitals, restaurants, hotels, cruise ships and many other applications. The test detects adenosine triphosphate – or ATP – a chemical found in all living things. ATP is present anywhere that people, bacteria, viruses, food, or other organic sources can be found and is present in the soil on surfaces that cleaning is designed to remove. The presence of ATP remaining on surfaces following cleaning is indicative of incomplete or improper cleaning.
ATP tests have become popular because they are easy to use and provide immediate results. The results from these traditional ATP tests, however, can often be deceiving in many applications because of an inherent weakness in their detection of ATP. A failure to understand this phenomenon can cause acceptance of false passing results and subsequently acceptance of faulty and ineffective cleaning.
The problem stems from the fact that in certain applications and under many environmental conditions the ATP molecule can degrade to adenosine diphosphate (ADP) and/or adenosine monophosphate (AMP).1 Traditional ATP tests can only detect ATP and will fail to detect these degradation products – even though their presence still indicates that the surface has not been adequately cleaned. But a test that could detect ATP plus the ADP and AMP that may have been generated will be far more sensitive and more effective for cleaning verification.
Recently, such a test that detects all three of these chemicals – ATP+ADP+AMP or “A3” – has been developed. The test was developed to make rapid ATP testing more useful in a wider variety of cleaning applications, including areas that have not previously employed cleaning verification, such as office building, retail, and other commercial facilities.
1 ATP, ADP, and AMP are also known as “adenylates’.
Cleaning Verification
Many high-risk facilities have long used verification of their cleaning processing as a crucial element of the safety programs. Hospitals, where ineffective cleaning can increase the risk of the transmission of healthcare-associated infections, and food processing plants, where cleaning is crucial to prevent bacterial contamination that can cause foodborne illnesses, have long used verification steps to measure their cleaning and sanitation effectiveness.These facilities had traditionally used visual inspection, but they learned that visual inspections alone are inaccurate, highly subjective, and ineffective in critical cleaning situations. These facilities long ago adopted ATP as a rapid method to measure and verify their cleaning programs.
Managers of commercial facilities should learn from these high-risk facilities and – like it or not – they are now largely in the same position of having to assure the cleanliness of their facilities to satisfy their employees, customers, and tenants of their safety.
Managers of high-risk facilities have also to come to understand the weaknesses of traditional ATP testing. A test that fails to accurately assess cleaning is not going to provide the data that you need to assure your stakeholders. You need a more sensitive test that more accurately detects residue that may be left behind.
Contamination from Hands
To demonstrate this phenomenon, we conducted a variety of scientific tests to investigate the sensitivity of traditional ATP tests compared with the improved A3 test along with their correlation to the removal of virus particles from surfaces.
It is well accepted that hand washing is an important step in preventing contamination and the spread of infections. This is true both for the protection of the person washing their own hands and to prevent the transfer of the contamination on their hands to high touch surfaces. Because people’s hands are the main source of contamination on high-touch surfaces, using soil from hands serves as a good proxy for a test to accurately detect that same debris present on those high-touch surfaces. To do this, tests to measure the contamination from the surfaces of hands, using gloved hand samples, were the first step in our evaluation .
Samples from gloved-hands were prepared by first having volunteers wash their hands using a commercially-available and commonly-used hand soap.2 The volunteers then wore powder-free, polyethylene gloves for 4 hours. At the end of that time period, 10 milliliters of sterilized water was added into each of their gloves to capture the soil from their hands. The liquid sample from the glove was then sampled and filtered. Feline calicivirus, a virus typically used as a surrogate for norovirus and effectively serves as a suitable proxy for tracking virus removal, was added to the sample.
Previous testing has shown that unwashed or ineffectively-washed hands will have an A3 RLU (relative light units- the units used in the measurement) measurement in a range of 5,000 to 50,000. The same research, using definitive laboratory tests for bacteria and organic debris, has established a standard of 2,000 RLU as a benchmark for”clean” hands. With this information, the volunteers washed their hand until a test with the A3 technology indicated that the debris on their hand was measured at 2,000 RLU or less.
Measuring High Touch Surfaces
To simulate the contamination of high-touch surfaces, this same soil- and virus-contaminated water was used to contaminate plastic sample coupons. 3
Each of 36 plastic sample coupons were contaminated in a 6 x 6 pattern of 11 microliter samples (“spots”). The plates were allowed to dry for 90 minutes to simulate commercial surfaces prior to cleanin g. Following drying, one of the plastic coupons was tested for ATP, A3 and the level of viruses present to represent baseline conditions pre-cleaning. Onadditional plastic coupons, each spot on the surfaces was wiped with a microfiber cloth. This was completed to represent a simple cleaning procedure. On additional plates, the surfaces were wiped four times, each time using a microfiber cloth to simulate a repeat cleaning procedure.
Following the initial and the repeat cleanings, testing of the surfaces was completed using two commercially-available, conventional ATP tests4, the A3 test, and a test for viruses. In this manner, the sensitivity of traditional ATP tests and the A3 test can be compared for sensitivity and also for their ability to correlate that sensitivity for removal of soil with their ability to serve as an indicator for the level viruses remaining on the surface after each cleaning. Separate plastic coupons were tested using the commercially-available ATP tests. A third plastic coupon was tested using the Kikkoman A3 test. All of the tests were performed according to the manufacturer’s instructions. A fourth plate was used to test for the level of viruses present using a standard cell culture plaque assay.5
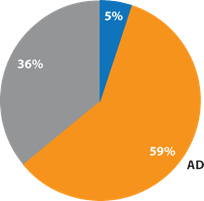
This response from the conventional ATP tests was to be expected. In previous examinations of samples from hands 6, it has been shown that the naturally-occurring levels of ADP and AMP are significantly higher than ATP (Figure 3). Since hands are the primary source of contamination on high-touch surfaces, this phenomenon is also seen in the proportion of adenylates on high-touch surfaces as measured in hospitals (Figure 4). Since ATP comprises only a small percentage of the total test target available, this places conventional ATP tests at a significant disadvantage.
The Kikkoman A3 test is capable of detecting these three adenylates simultaneously and this is evident in the level of response of the test. The ATP-only tests are unable to detect these adenylates and consequently show a significantly low RLU level, despite the fact that the surfaces are contaminated with organic debris and viruses.
Following a single wiping with the microfiber cloth, the level of virus had been reduced 96% but the Kikkoman A3 test still appropriately showed detectable levels of organic debris present. Both of the conventional ATP-only tests showed extremely low levels of ATP – lower, in fact, than the manufacturer’s recommended levels for effective cleaning. This is the effect mentioned earlier that conventional ATP tests can falsely indicate a clean surface due to their inability to detect the ADP and AMP still remaining from soils on the surface.
The data also shows that only after wiping the surface 4 times and producing a 99.5% reduction of the level of virus on the surface of the plate does the Kikkoman A3 test produce a RLU level below the manufacturer’s recommendations for effective cleaning. (See Table 1 for measured data from the tests).
Since ATP was the minor and ADP was the major adenylate in the gloved-hand samples, 20 times higher sensitivity in detection of debris from hands can be achieved by the A3 method, compared with the conventional ATP test.
– Journal of Preventative Medicine and Hygiene
3 Study conducted by Kanagawa Institute of Industrial Science and Technology in Japan
4 The commercially-available ATP assays were from 3M and Hygiena
5 In the plaque virus assay, susceptible stained host cells are spread and grown on a plate and inoculated with the sample containing the feline calicivirus . The feline calicivirus infects the host cells causing lysing , and the virus titer (proportional to the lysed cells) is measured in plaque-forming units.
6 Evaluation of the total adenylate (ATP+ ADP + AMP) test for cleaning verification in healthcare settings
M. BAKKE, S. SUZUKI, E. KIRIHARA, and S. MIKAMI Journal of Preventative Medicine and Hygiene, 2019, 60:E140- E146
ATP Test Conclusions
ATP hygiene monitoring tests can be used in commercial settings for verification of cleaning and sanitation because they are easy to use, provide immediate results for the verification of cleaning, and provide you with data you need to show your employees, tenants, and customers the care you are taking in your cleaning program to protect them. This study, however, demonstrates that conventional ATP tests can be ineffective in verifying cleaning procedures but a moreinnovative test, that employs a more comprehensive analysis that detects ATP, ADP, and AMP is a more effective means to measure surface cleaning.
As we have shown on high touch surfaces, where ATP is expected to account for a small percentage of total adenylate concentration, a test that detects ATP-only may produce misleading results. On the other hand, because the A3 test can detect three adenylates simultaneously, it provides a more sensitive and reliable indicator of cleaning effectiveness. And, while it is known that neither ATP nor the A3 test is specific for the direct detection of viruses, the additional sensitivity that the A3 test provides produces a more accurate measure of effective cleaning that has a proven high correlation with the removal of virus particles from surfaces. And, because the A3 test is just as easy to use as a less effective, conventional ATP testing , the A3 test is a preferred, advanced test for the verification of commercial cleaning.