COMET 8 Incubator Reader Starter Kit includes 25 Eclipse Farm 4G Tests
£495.00 ex. VAT
- Next generation Farm Incubator Reader for Antibiotics in Milk COMET8
- Test for All 8 Major Antibiotic Groups in 2.5hrs
- Test results emailed direct to your Inbox
- Simply add Milk Sample and Incubate
- Starter Kit Includes 25tests Eclipse FARM 4G
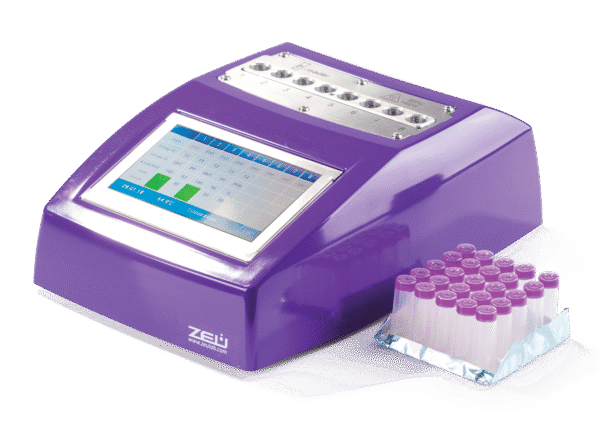
Comet8 Incubator Reader and Eclipse Farm 4G
One drop of milk, one minute of your time. For an automatic analysis of more than 50 antibiotic substances and with real-time results emailed to your inbox.
Do you want to make sure that the milk from your treated cow is free from antibiotics?
Do you have doubts when interpreting the color of microbial tests when detecting antibiotics in milk?
Sometimes you cannot stop the microbial assay at the right time and you doubt if your samples might contain antibiotics?
HOW DOES IT HELP YOU?
Eclipse FARM & COMET8 allow any operator to perform the analysis, receive the
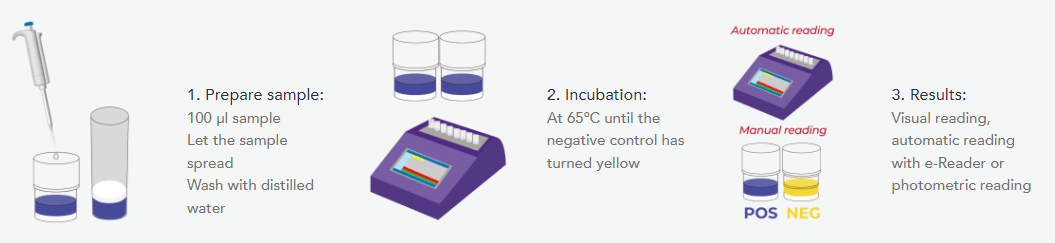
result wherever they are and share it in real-time. Just add a drop of milk into the test tube and Comet4 will automatically run the test in less than 2.5 hours. Regardless of where you are, you will receive the results on your smartphone, your cloud account or your email, always in real-time.
ECLIPSE test is based on the growth inhibition of the Geobacillus stearothermophilus bacterium. In a specific culture medium and under suitable conditions, the spores of this bacterium germinate and multiply, acidifying the medium, producing a colour change in the culture medium from blue to yellow. If the milk sample contains an antimicrobial concentration higher than the test detection limit, the bacterial growth is inhibited and therefore the colour change of the medium will not occur.
TECHNICAL DATA
- Scope: simultaneous detection of 8 groups of antibiotics in milk (>50 antibiotic substances). Sensitivity adapted to European MRLs
- Assay time: 2 hours 30 min.
- Automatic assay: adds the milk sample and automatic stop and interpretation of the results.
- Results: numerical results, automatic reading and results are automatically sent (WIFI/Bluetooth/4G).
- Format: 25 or 50 tubes.
Validations:
Eclipse Farm 4G & Comet 8 validated by the German laboratory MQD.
Validated by ILVO: Eclipse Farm 3G & Comet 8.
Internal validation: According to ISO 13969:2003.
Legislation:
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin.
COMET 8 Incubator and Reader for ECLIPSE FARM - 8place
| Weight | 1000 kg |
|---|