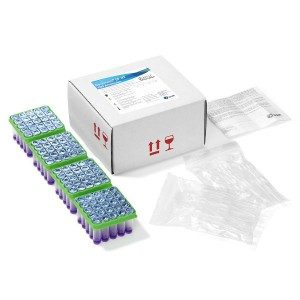
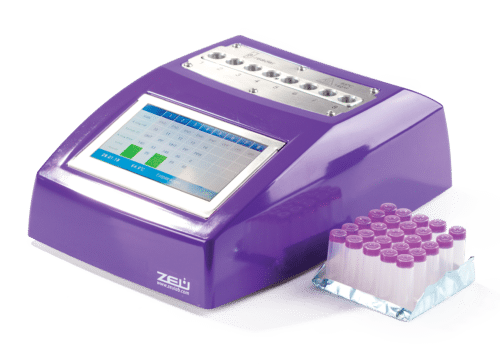
COMET Incubator – Reader for testing On-Farm Antibiotics
Please request a quote for the price.
S/n
Android Version
COMET Incubator – Test Principle
ECLIPSE test is based on the growth inhibition of the Geobacillus stearothermophilus bacterium. In a specific culture medium and under suitable conditions, the spores of this bacterium germinate and multiply, acidifying the medium, producing a colour change in the culture medium from blue to yellow. If the milk sample contains an antimicrobial concentration higher than the test detection limit, the bacterial growth is inhibited and therefore the colour change of the medium will not occur.

- Scope: simultaneous detection of 8 groups of antibiotics in milk (>50 antibiotic substances). Sensitivity adapted to European MRLs
- Assay time: 2 hours 30 min.
- Manual assay: one-stage incubation at 63°C for approximately 2.5h and visual reading.
- Results: numerical results, automatic reading and results are automatically sent (WIFI/Bluetooth/4G)
- Portable: 4 hours battery life
- Format: 25 or 50 tubes
Validations:
Eclipse Farm 4G & Comet were approved by the AOAC-RI Performance Tested Methods (PTM) Program for 29 antibiotics. (PTM License Number 022101)
Validated by ILVO: Eclipse Farm 4G & Comet4 fulfils the Belgian FASFC approval criteria regarding detection capabilities.
Internal validation: according to ISO 13969:2003 and CRL guides (Community Reference Laboratory for veterinary residues), Commission Decision 2002/657/EC.
Legislation:
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin.